- 1School of Agriculture, Fertilization, and Environmental Sciences (ESAFE), Mohammed VI Polytechnic University, Ben-Guerir, Morocco
- 2AgroBioSciences, Mohammed VI Polytechnic University, Ben-Guerir, Morocco
- 3African Sustainable Agriculture Research Institute (ASARI), Mohammed VI Polytechnic University (UM6P), Ben-Guerir, Morocco
- 4Department of Pharmacology and Toxicology, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
Medicinal plants have been used since ancient times for human healthcare as drugs, spices, and food additives. The progress in technology and medicine observed, the last decades, has improved the quality of life and healthcare but with worrisome drawbacks. Side effects caused by synthetic drugs for instance originate sometimes irreversible health disorders. Natural substances, in contrast, are biologically and environmentally friendly. Syzygium jambos L. (Alston) also known as rose apple conveys a long history as essential traditional medicine with a broad spectrum of application in various cultures. The plant discloses a diverse group of secondary metabolites and extracts that displayed major susceptibilities towards various health concerns especially stress-related and inflammatory diseases. Despite a rich literature about the plant, the chemistry and biology of S. jambos have not been comprehensively reviewed yet. Accordingly, we present herein a literature survey of rose apple which aims to draw the chemical identity of the plant and establish a consistent discussion on the respective biological application of plant extracts and their corresponding traditional uses. The present work could provide a scientific basis for future studies and necessary information for further investigations of new drug discovery.
Introduction
The renown of alternative medicines nowadays is appealing although progress in technology and medicine encountered the last decades has improved the quality of life and healthcare around the world. Corresponding drawbacks are quite worrisome. Side effects caused by synthetic drugs for instance hurt human health system, sometimes with irreversible impacts (van Wyk and Wink 2015). Natural substances, in contrast, are biologically and environmentally friendly as they are recognized by other organisms which facilitate their metabolisms. These substances are provided from plants, microorganisms, or animals with a pronounced interest since they constitute the main sources of foods and thus, our first resort in case of pain (van Wyk and Wink 2015).
Plants contain chemicals not essential for their metabolism rather for the fight against attacks and stress due to the plant habitats. These phytochemicals have shown distinct biological properties against numbers of illnesses (Iwu, 1993; van Wyk and Wink 2015). Both plants and compounds are of great interest in drug development to face new medical challenges.
Accordingly, numerous of research works have been conducted on plants from the genus Syzygium to elucidate its chemistry and pharmacology. Species of this genus, including S. jambos, offer edible fruits found under various formulation including juices, jellies, and jams (Sun et al., 2020). The decoction of these fruits serves to alleviate gastrointestinal disorders, wounds, syphilis, leprosy, as well as toothache (Chua et al., 2019). Reports have highlighted the occurrence of polyphenols, flavonoids, tannins, and sterols from various organs of S. jambos species. Meanwhile, plant extracts and compounds also claimed a broad spectrum of activities from antibacterial to anti-inflammatory activities through analgesic, antiviral, anti-dermatophyte, anticancer, and hepatoprotective properties (Sobeh et al., 2018). Two recent reviews very briefly highlighted the chemical composition, traditional uses and biological activities of the plant (Harsha et al., 2021; Subbulakshmi et al., 2021).
The present research survey tends to summarize the traditional uses, chemical constituents, and pharmacological properties of extracts and compounds from S. jambos in one document as much information as possible about this plant, which has many biological properties. This work could provide a scientific basis for future study and necessary information for further investigations of new drug discovery.
Taxonomy and Botanical Description
The genus Syzygium contains approximately 1,200–1800 species, the majority of which are flowering plants (Khalaf et al., 2021). Its taxonomy has been disputed for long with that of the genus Eugenia (Mabberley, 2017). As a result, species of the later have been ranged in the genus Syzygium. Amongst them, S. malaccence, S. suborbiculare, S. paniculatum, S. aqueum, samarangense, and S. jambos (Sobeh et al., 2016; Cock and Cheesman, 2018). S. jambos L. Alston, synonym of Eugenia jambos, is native to Reunion Island, Central America (Guatemal), and South-East of Asia, especially in Nepal, Indonesia, Philippines, and Malaysia. It has been naturalized in India and claims various vernacular names in different cultures including malabar plum, plum rose, rose apple, and water apple (Maskey and Shah, 1982; Morton, 1987; Avila-Peña et al., 2007).
S. jambos belonging to the family Myrtaceae, is a medium sized tree reaching 7.5–12 m in height, Figure 1 (Morton, 1987). Due to its physical characteristics and the aroma of the fruits, the plant is often known as rose apple. It has a dense crown of slender with wide spreading branches. Leaves are opposite, lanceolate, and glabrous with 2.5–6.25 cm wide and 10–22 cm length. They are glossy and dark-green when mature while vibrant red when young. Flowers are in small terminal clusters, white or greenish white with a diameter of 5–10 cm. Usually, there are 4–5 flowers together in terminal clusters (Nawwar et al., 2016). The berries have a fleshy pericarp with 10–15 mm thick on the tree. They are sub-globose and whitish-to pinkish-yellow color. Every fruiting season, a mature rose apple tree produces about 35.57 g of fruit, with 7.16 cm length and 5.15 cm width. The epicarp of the fruit is thin, smooth, and reddish, while the mesocarp and endocarp are whitish and succulent,Figure 1 (Daly et al., 2016; Mangini et al., 2020).
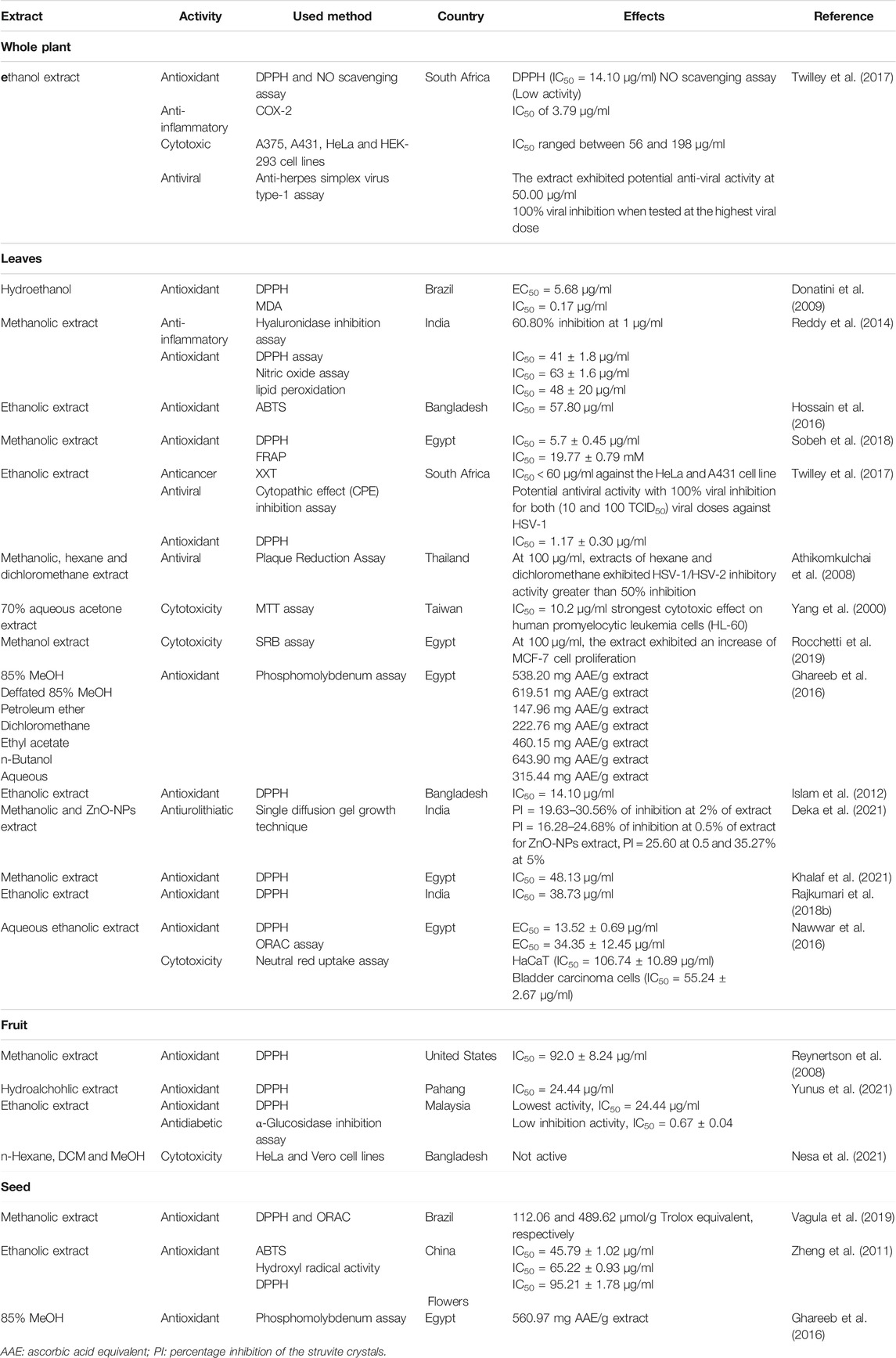
Phytochemical Composition
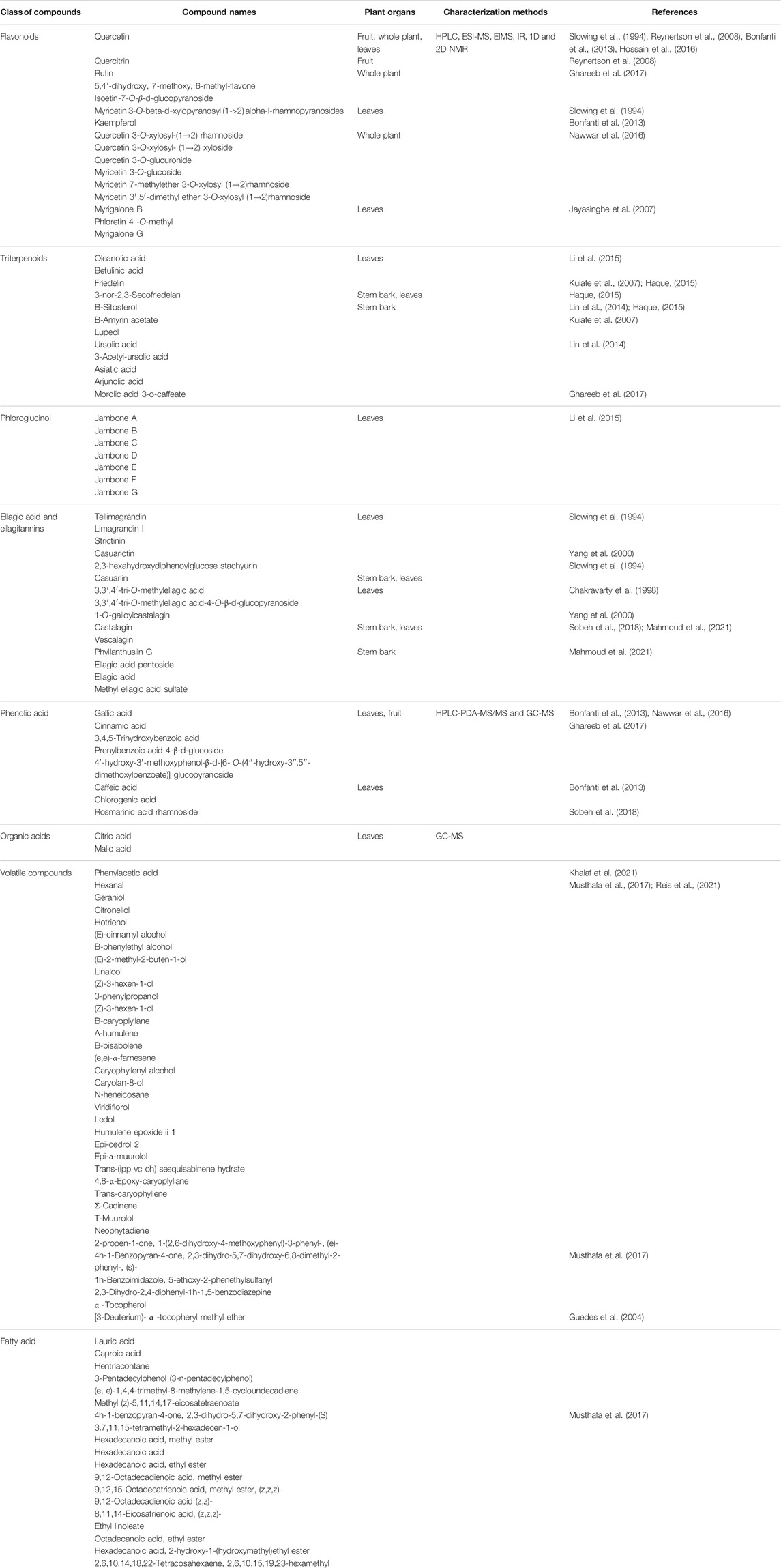
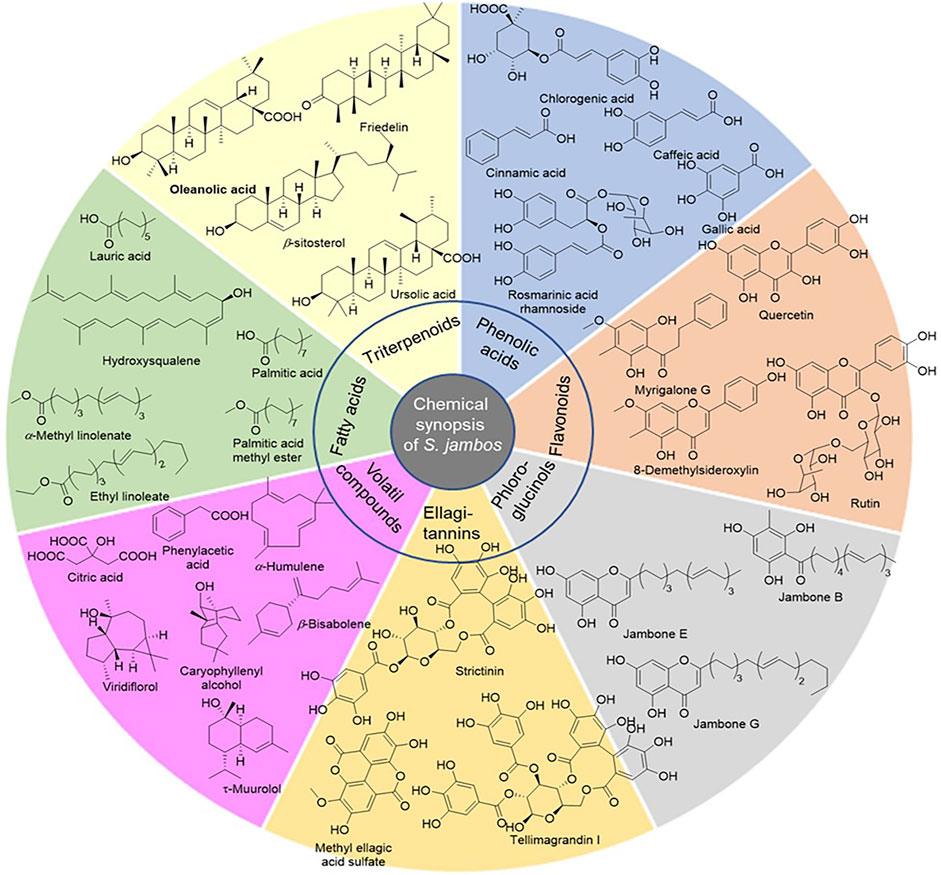
Phenolic compounds are mainly present in the leaves of S. jambos. They are represented by flavonoids, ellagitannins, phloroglucinols, and phenolic acids, Table 1; Figure 2 (Rocchetti et al., 2019; Slowing et al., 1994; Slowing et al., 1996; Sobeh et al., 2018). Flavonoids are the most abundant group of compounds while quercetin sounds to be the most abundant monomer in every organ of the plant, except the stem bark. It is found in both aglycone and saponin forms. Only flavone and chalcone-types of flavonoids occur in S. jambos (Reynertson et al., 2008). Some anthocyanidins have also been detected in the plant mainly, petunidin 3-O-glucoside, pelargonidin 3-O-(6″-malonyl-glucoside) and delphinidin 3-O-galactoside (Rocchetti et al., 2019). Catechin has been identified from the leaves of the plant suggesting a tentative occurrence of non-hydrolysable tannins in the plant. As part of tannins, only ellagitannins (hydrolysable tannins) have been found in some plant extracts to date. Likewise, ellagic acid monomer derivatives have also been reported in the leaves and stem bark of the plant. Moreover, phenolic acids, listed as intermediates in the metabolism of flavonoids and ellagic acids like gallic acid and cinnamic acid, have also been alarmed in the leaves and fruit of S. jambos. Gallic acid is the most abundant and distributed phenolic acid in the plant. The other phenolic acids were either glycosylated benzoic acid or derivatives of phenylpropanoids. Phloroglucinols also occur in S. jambos leaves. Though only one report highlighted their presence in S. jambos, phloroglucinols are well distributed in Myrtaceae family. The seven compounds of this class were isolated from a Chinese species and no trace of one of this group of compounds was mentioned in the Egyptian or Brazilian varieties, Table 1; Figure 2 (Li et al., 2015).
Pentacyclic triterpenoids are also abundant in the plant especially in the leaves and stem bark. They belong to oleanane, ursane, lupane and friedelane subclasses. The major ones were betulinic acid and friedelin. Saponins of triterpenes have not yet been isolated except the readily available β-sitosterol glucoside, Table 1 (Kuiate et al., 2007; Li et al., 2015). Roots and flowers of the plant have not been investigated yet.
The essential oil of the plant leaves contain mostly volatile sesquiterpenes including δ-cadinene, cumaldehyde, β-himachalene, isocaryophyllene, and β-cedrene, Table 1 (Khalaf et al., 2021). Linalool is one of the essential oil markers in the identification of the plant fruit. Indeed, linalool, cinnamyl alcohol, and geraniol are the main volatile terpenes in the extracts. Differences were observed in the volatile aromatic composition of fruits from the Brazilian, Malaysian, and Egyptian species. Linalool was found as the main compound in the Brazilian fruits while 3-phenylpropyl alcohol (Z)-3-hexen-1-ol and (Z)- cinnamaldehydes were identified as major compounds in the Malaysian and Egyptian ecospecies (Vernin et al., 1991; Wong and Lai, 1996; Guedes et al., 2004; Ghareeb et al., 2017).
Traditional Uses
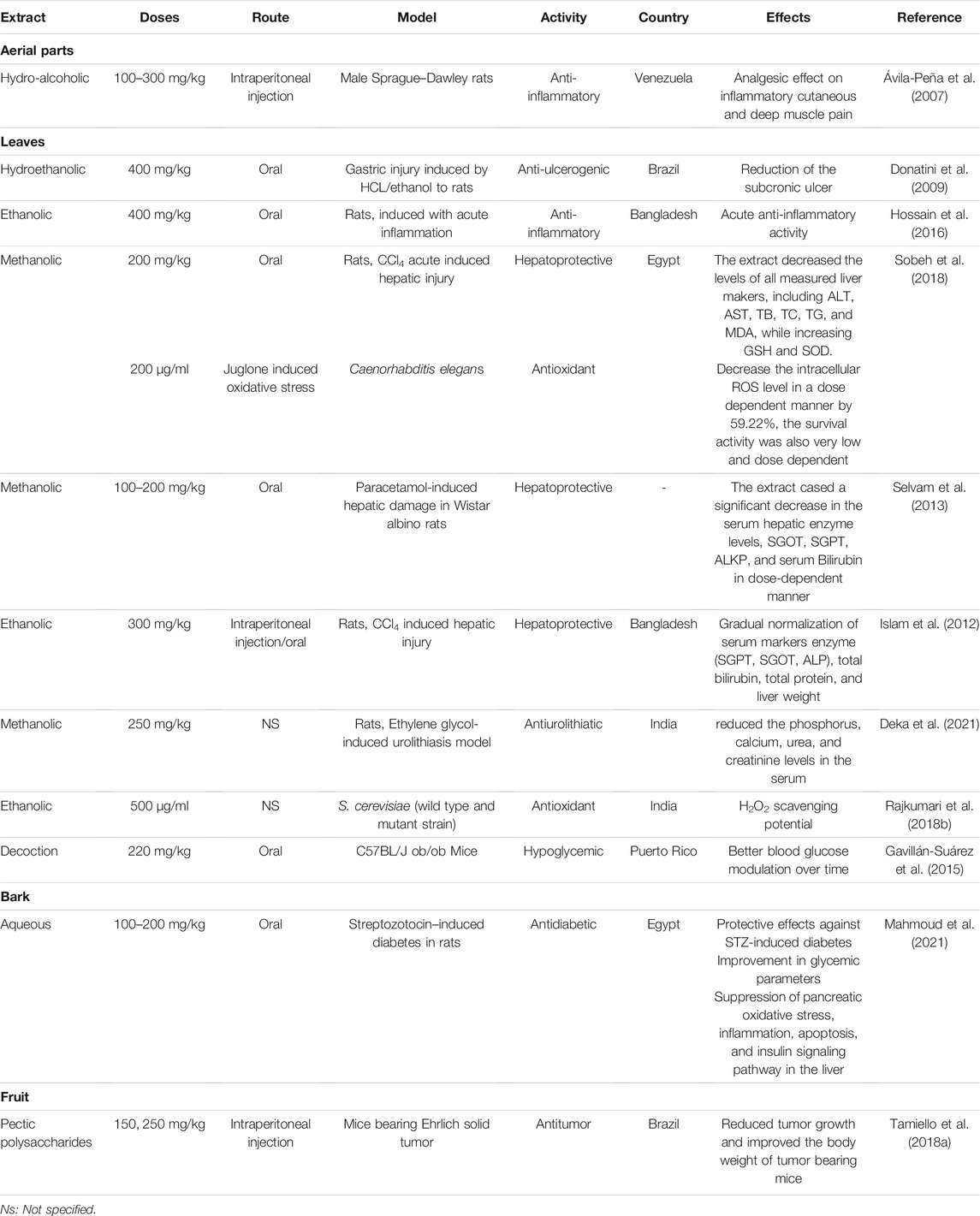
Rose apple carries a long history as essential traditional medicine with a broad spectrum of application in various cultures. In India, the fruit tonic helps to improve brain and liver health while fruit infusions convey diuretic property (Morton, 1987). Moreover, the juices from macerated leaves in water were used as a febrifuge (Maskey and Shah, 1982). Dysentery is also alleviated by the seeds together with diarrhea, and catarrh. Furthermore, the flowers are assumed to relieve fever (Baliga et al., 2017). The infusion of the powdered leaves is beneficial to diabetes (Maskey and Shah, 1982). In South American cultures, the seeds have an anesthetic property whereas leaf decoction is applied to sore eyes, and used as diuretic, expectorant and to treat rheumatism (Maskey and Shah, 1982). The decoction of the bark is administered to treat asthma, bronchitis, and hoarseness (Maskey and Shah, 1982). The plant is also used to treat hemorrhages, syphilis, leprosy, wounds, ulcers, and lung diseases due to its potency to relieve fever and pains. In China, each plant organ is used to treat digestive tract and tooth pains (Mahmoud et al., 2021; Reis et al., 2021).
Biological Activities
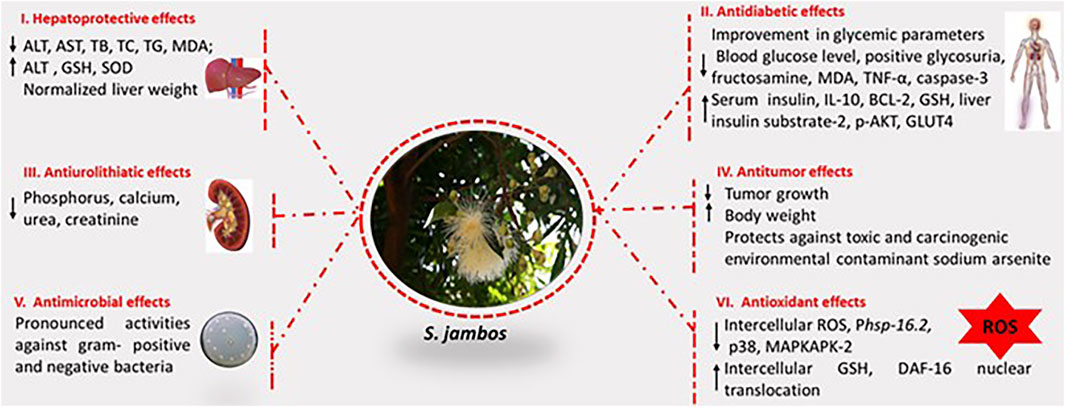
The biological applications of S. jambos are rich and diverse. Isolates were screened in accordance with the traditional uses of the plant encountered worldwide. Mainly, plant extracts and compounds have presented antifungal, antibacterial, hepatoprotective, analgesic, antioxidant, anti-inflammatory, antidiabetic, anticancer, anti-pyretic activities, Figure 3. The main pharmacological characteristics of S. jambos are listed in Tables 2–4.
Toxicity Studies
To date, only few literatures have reported the toxicity of the plant. The leaf extract of S. jambos is safe at a dose up to 5 g/kg b.wt. assessed by the acute toxicity test (Dhanabalan and Devakumar, 2014). The toxicity of the methanol extract of S. jambos and its fraction were evaluated by shrimp lethality bioassay. Methanolic extract and carbon tetrachloride fraction displayed significant lethality with LC50 = 6.97 and 13.61 µg/ml, respectively. Whereas the chloroform and hexane fractions showed moderate to low lethality with LC50 = 64.94 µg/ml and 257.6 µg/ml, respectively (Haque, 2015). In the same line, Ghareeb et al. (2016) tested different extracts and fraction obtained from the leaves and flowers against the brine shrimp Artemia salina, a useful tool to determine the toxicity of natural products. As a result, the n-butanol fraction of the leaves showed a strong toxicity with LC50 = 50.11 µg/ml while the dichloromethane and petroleum ether fractions were less toxic (LC50 = 446.65 µg/ml) (Ghareeb et al., 2016).
Toxicology safety evaluation is essential for plants applications and new drug development. However, the toxicological studies of extracts and compounds isolated from S. Jambos have not been fully explored yet. Therefore, further research in toxicity is needed to determine the suitability of the plant extracts and related compounds composition.
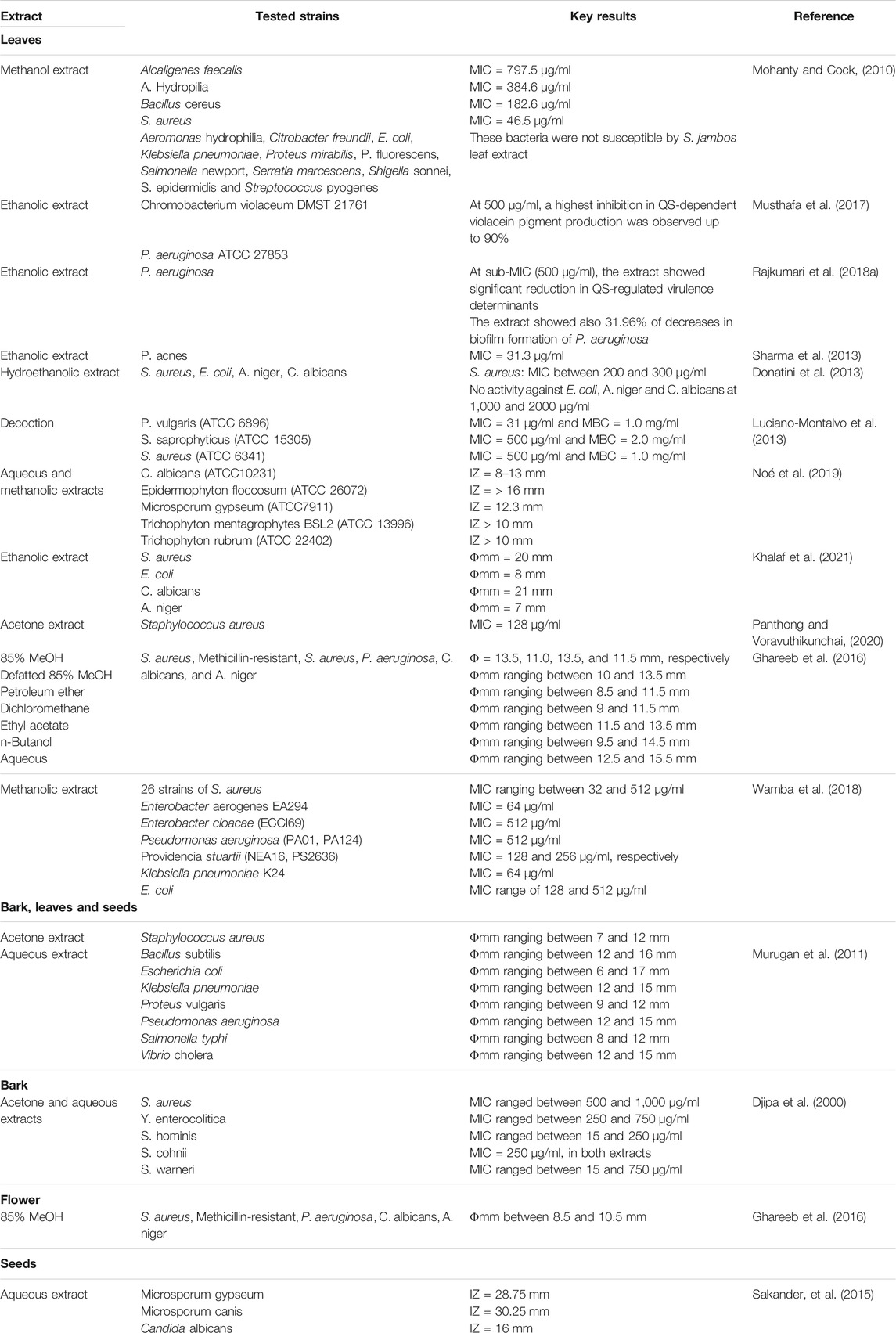
Antimicrobial Activity
Diverse antimicrobial activity of crude extracts and isolated compounds from the plant were described in previous reports. Disc diffusion assays, agar well diffusion, and broth microdilution procedures were employed to assess the antibacterial activity of plant extracts. As shown in Table 2. Microbial growth inhibition zones and percentages, as well as minimum inhibitory concentrations (MICs), demonstrated that S. jambos has potential as a significant antibacterial agent.
Wamba et al. (2018) reported the capacity of S. jambos extracts to increase the potency of chloramphenicol antibiotic towards bacteria strains expressing MDR phenotype (Wamba et al., 2018). Leaf and bark extracts of the plant expressed up to 70% of antibiotic-modulating activity against S. aureus strains at MIC/2. Similar results were obtained in association with tetracycline, ciprofloxacin, and erythromycin against Gram-negative bacteria including strains of Escherichia coli (AG100ATet, AG102), Enterobacter aerogenes (EA27, EA289), Klebsiella pneumoniae (KP55, KP63), Providencia stuartii (PS299645, NEA16) and Pseudomonas aeruginosa (PA01, PA124) (Wamba et al., 2018). Likewise, S. jambos leaf extracts demonstrated potent antiviral effects on the virus involved in vesicular stomatitis and against different types of herpes simplex virus (Abad et al., 1997; Athikomkulchai et al., 2008).
Isolated compounds friedelin, β-amyrin acetate, betulinic acid, and lupeol, from the bark extract, were tested for their antidermatophytic activity against three commonly dermatophyte species found in Cameroon namely Microsporum audouinii, Trichophyton mentagrophytes and T. soudanense. Betulinic acid and friedelolactone were the most active compounds with MIC ranging from 12.5 to 100 µg/ml and the most sensitive fungi were Trichophyton soudanense (MIC = 25 µg/ml) and Trichophyton mentagrophytes (12.5 µg/ml) (Kuiate et al., 2007). The phenolic compounds, quercetin, rutin, prenylbenzoic acid 4-O-β-D-glucopyranoside, morolic acid 3-O-caffeate, 5,4′-dihydroxy-7-methoxy-6-methylflavone, 3,4,5-trihydroxybenzoic acid, isoetin-7-O-β-D-glucopyranoside, and (4′-hydroxy-3′-methoxyphenol-β-D-[6-O-(4″-hydroxy-3″,5″-dimethoxylbenzoate)] glucopyranoside) also exhibited both antibacterial and antifungal potentials with a diameter of inhibition zones ranging from 9–19 mm (Ghareeb et al., 2017). Accordingly, the antimicrobial activity of S. jambos crude extracts have been related to the presence of an increased level of tannins in the preparation (Baliga et al., 2017).
Moreover, silver nanoparticles synthetized from leaves and bark extracts of S. jambos showed higher antiplasmodial activity against chloroquine sensitive and resistant strains of Plasmodium falciparum (Dutta et al., 2017). The fatty compounds, ethyl linoleate, methyl linolenate and phytol, inhibited the QS-dependent pigment production in C. violaceum and lowered pyoverdine production in P. aeruginosa as well. Results were also confirmed by docking analysis (Musthafa et al., 2017). The above research confirmed the antimicrobial activity of S. jambos. However, it is worthy to note that the above studies focused on the in vitro evaluations. Consequently, these studies only give preliminary information about the activity of S. jambos. Therefore, further studies combining in vivo and in vitro need to be conducted to provide reliable basis for exploring new potentially and low toxic antimicrobial agents from the studied plant.
Antioxidant Activity
Several studies, both in vitro and in vivo, reported the antioxidant activity of S. jambos extracts and its phytochemicals. Bonfanti et al. (2013) demonstrated the potency of the leaf aqueous extract of S. jambos to inhibit the nitric oxide radical, the lipid peroxidation and the mitigation sodium-nitroprusside-induced oxidative stress in rats. The extract also showed a capacity to increase the GSH levels in rats (Sobeh et al., 2018). Furthermore, the bark extract inhibited lipid peroxidation and increased reduced glutathione (GSH) in pancreatic tissues of STZ-diabetic rats (Mahmoud et al., 2021). S. jambos leaf extract abolished ROS production by endothelin-1 in human polymorphonuclear and mononuclear cell migration (Inostroza-Nieves et al., 2021). On the other hand, S. jambos rich phenolic and flavonoid fractions demonstrated good antioxidant activities as shown in Table 3. The chalcones phloretin 4′-O-methyl ether, myrigalones B and G were assessed for their antioxidant activity using DPPH radical. As a result, myrigalone B showed a significant capacity of scavenging radicals with an IC50 of 3.8 µg/ml while the other compounds showed low to moderate activity (IC50 > 30 µg/ml) (Jayasinghe et al., 2007). Moreover, 2,6-dihydroxy-4-methoxy-3,5-dimethyldihydrochalcone showed anti-DPPH activity with an IC50 value of 10.6 µg/ml while, the flavones, 4′-methoxysideroxylin and 6-demethylsideroxylin, and phloroglucinols, jambones A-B, presented weak antioxidant activities in FRAP and DPPH radical scavenging activities (Li et al., 2015).
Neurological Activity
There are relatively few studies on neuroprotective effect of S. jambos. Bonfanti et al. (2013) investigated the effects of S. jambos in the inhibition of both AChE and BuCE, the two main enzymes in the occurrence of Alzheimer. As a result, the aqueous leaves extract of S. jambos showed significant AChE (IC50 = 16.5 µg/ml) and BuCE (IC50 = 15.2 µg/ml) inhibition potentials in support with the uses of the plant to alleviate Alzheimer disorders. Considering these findings, further investigations may improve the neuroprotective effect of S. jambos.
Anticancer Activity
In vitro anticancer activity of isolates from S. jambos was determined towards various cancer cell lines, providing data on the bioactivity of both extract and single compounds, Table 3. Methanolic extract of S. jambos leaves showed cytotoxic effects against liver cancer cell line, Hep G2 cells, by inducing apoptotic pathways (Thamizh Selvam et al., 2016). Moreover, another study evaluated the anticancer effects of the leaves along with other extracts on human melanoma (A375), epidermoid carcinoma (A431), cervical epithelial carcinoma (HeLa) and human embryonic kidney cells (HEK-293). They found that the extract showed low toxicity against HEK-293 cells but better effects against A431 and HeLa cells (IC50 = 34.90–56.20 μg/ml) (Twilley et al., 2017). The hydrolysable tannins, 1-O-galloyl castalagin and casuarinin, exhibited significant cytotoxic activity against the human promyelocytic leukemia cell line HL-60 with IC50 of 10.8–12.5 µM and showed moderate to low cytotoxicity on the human adenocarcinoma SK-HEP-1, normal cell lines of human lymphocytes and liver cell lines. Results were confirmed by DNA fragmentation assay and microscopic investigation of cells (Yang et al., 2000). The cytotoxic effects of the phenolic compounds, cis-3-p-coumaroylalphitolic acid and 4′-methoxysideroxylin, on melanoma SK-MEL-28 and SK-MEL-110 cell lines were assessed as well as that of the normal Vero cells, following the MTT assay. The compounds, displayed potent effects on the two melanoma cells with IC50 ranging from 18.3–81.5 µM (Li et al., 2015). The cytotoxic effect of quercetin-3-O-β-D-xylofuranosyl-(1 → 2)-α-L-rhamnopyranoside and myricetin-3-O-β-D-xylofuranosyl-(1 → 2)-α-L-rhamnopyranoside isolated from the CH2Cl2/MeOH fraction of the plant was evaluated against RW 264.7 cell lines. Both flavonoids demonstrated a moderate activity (IC50 = 1.68 and 1.11 µM, respectively) (Ticona et al., 2021). The cytotoxic effect of the nanoparticles synthetized from the leaf and bark extracts of S. jambos was assessed against HeLa and L6 cells using MTT assay. As a result, the nanoparticles were found to be non-toxic toward HeLa and L6 cell lines (Dutta et al., 2017). These investigations provided the anticancer potential of S. jambos, further in vivo, toxicological, and clinical studies are needed in future to guarantee efficiency and safety.
Anti-Inflammatory Effect
Inflammation and specifically low-grade inflammation play a vital role in many diseases. Natural products with anti-inflammatory effects are promising targets for drug discovery. In vitro and in vivo models were applied to determine the anti-inflammatory effects of crude extracts and pure compounds from S. jambos. In vitro studies showed that the ethanol leaf extract of S. jambos and the commercially available chemicals ursolic acid and myricitrin dramatically reduced the release of inflammatory cytokines IL 8 and TNF-α by 74–99% indicating anti acne effects (Sharma et al., 2013). A more recent study on two isolated glycosylated flavonoids, the quercetin-3-O-β-D-xylofuranosyl-(1 → 2)-α-L-rhamnopyranoside and myricetin-3-O-β-D-xylofuranosyl-(1 → 2)-α-L-rhamnopyranoside, isolated from the chloroform/methanol fraction of S. jambos showed that they reduced the production of TNF-α, with IC50 values of 1.68 and 1.11 M, respectively in the RAW 264.7 cell line. In addition, at a dose of 5 mg/kg, the flavonoids reduced the levels of TNF-α, C-reactive protein, and fibrinogen in murine models (Apaza Ticona et al., 2021). In vivo studies showed that the ethanol extract of the leaves also exerted potent anti-inflammatory effects at a dose of 400 mg/kg in carrageenan and histamine edema rat models (Hossain et al., 2016). The soluble fraction of polysaccharide fraction of the plant also expressed a capacity to increase the secretion of TNF-α, IL-1β and IL-10 in a concentration-dependent manner (10–100 µg/ml). The aqueous extract of the plant attenuated the inflammatory response induced by LPS at a concentration of 100 µg/ml (Tamiello et al., 2018b). Furthermore, the bark extract inhibited pancreatic inflammation in STZ diabetic rat model where it dose-dependently suppressed the pro-inflammatory, TNF-α and increased the anti-inflammatory IL-10 levels (Mahmoud et al., 2021).
Hepatoprotective Activities
Liver is one of the largest and important organs in human body and performs numerous interrelated vital functions, such as metabolism, biotransformation, and detoxification of toxins. Consequently, liver diseases resulting from liver damage is a global problem. Herbal medicine has been used traditionally for the prevention of liver diseases (Islam et al., 2012). Preclinical studies have shown that extracts from different parts of S. jambos possess beneficial effect in liver related diseases, Table 4. The methanol extract of the leaves of the plant significantly modulated the levels of liver biochemical parameters ALT, AST, MDA, TB, TC, TG, GSH and SOD) in comparison with the positive control, silymarin, Table 4 (Sobeh et al., 2018). Isolation of the compounds of the extract may led to the discovery of promising active constituents.
Antidiabetic Activity
Diabetes and diabetic complications are global health problem. Although many medicinal plants were investigated for their possible antidiabetic activities, there are relatively few studies on antidiabetic effect of S. jambos extracts. An in vitro study compared the inhibitory effects of ethanol extract of different organs of S. jambos on α-glycosidase and α-amylase activities, enzymes related to diabetes, and showed that the inhibitory effects against yeast and mice intestinal α-glucosidase activity was on the following order: seed ˃ stem ˃ leaf ˃ root ˃ flower ˃ flesh ˃ acarbose, while the inhibitory effect on α-amylase activity was acarbose ˃ seed ˃ stem ˃ root ˃ leaf ˃ flesh ˃ flower (Wen et al., 2019). In vivo studies showed that the infusion of the combined leaves of S. jambos and S. cumini had no significant effect on blood glucose levels in a randomized double-blind clinical trial in non-diabetic and diabetic subjects (Teixeira et al., 1990). However a more recent study showed that the ethanol extract of leaves at two dose levels (374.5 mg/kg and 749 mg/kg, Po) lowered blood glucose levels in alloxan induced diabetic rabbits (Prastiwi et al., 2019). Moreover, an aqueous leaf extract from the plant showed better blood modulation potential of glucose over time, in diabetes genetic mouse models (Gavillán-Suárez et al., 2015). Recent studies have shown the protective effect of the bark extract on pancreatic β cells against streptozotocin-induced diabetes. The extract have also improved insulin signaling pathway in the liver and glycemic parameters and have suppressed pancreatic oxidative stress (Mahmoud et al., 2021). However, further studies need to be conducted to confirm the potential of S. jambos as a natural antidiabetic agent, as it can be incorporated into functional foods and nutraceutical products.
Antiurolithiatic Activity
The antiurolithiatic activity of the leaf extract of S. jambos, collected in India, was evaluated both in vitro and in vivo using ethylene glycol induced urolithiatic model in rats. Results showed a capability of the extract to prevent the growth of urinary stones. However, further studies should be done to understand the mechanism and pharmacological action in preventing urolithiasis in susceptible populations (Deka et al., 2021).
Discussion
The main chemicals found in S. jambos were phenolic compounds and triterpenoids. Phenolic compounds were the major constituents of the plant. They are made up of glycosylated flavonoid and ellagitannin derivatives. Plant extracts showed significant antibacterial activity, improving the potency of strong antibiotics like tetracycline, ciprofloxacin, erythromycin, or chloramphenicol. Likewise, both water-soluble fraction and organic extracts have shown significant capabilities in reducing radicals and heavy metal ions. In vivo anti-inflammatory activity of plant extracts has also been demonstrated with considerable endpoints. These biological characteristics of the plant could be related to their main chemical constituents. Flavonoids and ellagitannins are excellent free radical scavengers (Koagne et al., 2020). For this reason, they protect cells from aging and stress, and exerted anti-nociceptive activities. Indeed, S. jambos plant extracts have shown considerable anti-inflammatory activity towards some models. The analgesic potential has been ascribed to two glycosylate flavonols occurring in rose apple namely, myricetin-3-O-β-D-xylofuranosyl-(1 → 2)-α-L-rhamnopyranoside and quercetin 3-O-β-D-xylopyranosyl-(1→2)-α-L-rhamnopyranoside. However, no mechanism of action of the recorded biological activity was proposed yet. Nevertheless, both antioxidant and anti-inflammatory activities encountered for S. jambos extracts and compounds are closely related. The anti-inflammatory potency of rose apple extracts is a key point in the uses of plant extracts to alleviate different illnesses. More importantly, the major constituents of S. jambos extracts, flavonoids and ellagitannins, are mostly glycosylated. They can then be found in large extent in the blood because of their water solubility. This parameter is quite important in drug development as it improves the therapeutic action of a drug. Accordingly, S. jambos constitutes a potential candidate to the development of potent traditional drugs against ROS and inflammation-induced illness.
Conclusion and Perspectives
This review provides an up-to-date summary of S. jambos from the perspectives of its phytochemistry, pharmacology, traditional uses as well as toxicology. Phytochemical investigations have been focused on different organs of the plant, prepared with various organic and water solvents. These studies revealed the presence of flavonoids (flavones, chalcones, anthocyanins and proanthocyanins), ellagitannins, phenolic acids, triterpenoids, volatiles compounds and fatty analogues. Compounds were either isolated following chromatographic techniques or identified by online methods like HPLC-MS/MS and GC-MS. Flavonoids and saponins as well as phenolic acids are the main constituents of the plant.
Activities of the plant towards pathogens and cells are also diverse and rich, consecutive to the broad spectrum of applications of the plant in traditional medicine to alleviate some illnesses. Plant extracts showed considerable anti-inflammatory activity and a synergistic effect to antibiotics activity of some popular drugs correlating the uses of the plant to relieve pains and infection. Extracts have also antiviral, anti-dermatophyte, hepatoprotective, and anticancer effects. Numerous compounds were isolated and initially screened for their bioactive potential. Further investigations are needed to complete the phytochemical profile, pharmacology mechanisms and pharmacokinetics studies of the plant. In the same line, toxicity study of S. jambos is indispensable in the future to assess the safety of the plant and its bioactive compounds to support possible future medicinal applications and before proceeding to the development of pharmaceutical formulations.
Author Contributions
MAO and WBB drafted the manuscript; GTMB and MFM reviewed the manuscript; MS revised the manuscript and designed and conceived the study. All authors approve the final version.
Funding
The APC was paid by UM6P.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
A375, Human melanoma cancer cell line; A431, Epidermoid carcinoma cancer cell line; AChE, Acetylcholinesterase; ALA, Artemia lethality assay; BuCE, Butyrylcholinesterase; COX-2, Cyclooxygenase-2 inhibition assay; DNA, Deoxyribonucleic acid; HEK-293, human embryonic kidney cells; HeLa, Cervical epithelial carcinoma; L6, Rat skeletal muscle cell line; MCF-7, Human breast cancer cell line; MDA, Malondialdehyde; MDR, Multidrug resistance; MIC, Minimum inhibitory concentration; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; ROS, Reactive oxygen species; SRB, Sulforhodamine-B; WSP, Water soluble polysaccharides; XTT, 2,3-Bis-(2-methoxy-4-nitro-5- sulfophenyl]-2Htetrazolium-5-carboxyanilide salt; ZOI, Zone of infection.
References
Abad, M. J., Bermejo, P., Villar, A., Sanchez Palomino, S., and Carrasco, L. (1997). Antiviral Activity of Medicinal Plant Extracts. Phytother. Res. 11 (3), 198–202. doi:10.1002/(sici)1099-1573(199705)11:3<198:aid-ptr78>3.0.co;2-l
Apaza Ticona, L., Souto Pérez, B., Martín Alejano, V., and Slowing, K. (2021). Anti-inflammatory and Anti-arthritic Activities of Glycosylated Flavonoids from Syzygium Jambos in Edematogenic Agent-Induced Paw Edema in Mice. Rev. Bras. Farmacogn. 31 (4), 429–441. doi:10.1007/s43450-021-00167-0
Athikomkulchai, S., Lipipun, V., Leelawittayanont, T., Khanboon, A., and Ruangrungsi, N. (2008). Anti-herpes Simplex Virus Activity of Syzygium Jambos. J. Health Res. 22 (1), 49–51.
Ávila-Peña, D., Peña, N., Quintero, L., and Suárez-Roca, H. (2007). Antinociceptive Activity of Syzygium Jambos Leaves Extract on Rats. J. Ethnopharmacology 112 (2), 380–385. doi:10.1016/j.jep.2007.03.027
Baliga, M. S., Ranganath Pai, K. S., Saldanha, E., Ratnu, V. S., Priya, R., Adnan, M., et al. (2017). “Rose Apple (Syzygium Jambos (L.) Alston),” in Fruit and Vegetable Phytochemicals: Chemistry and Human Health. Editor E. M. Yahia. Second Edition 2 (Hoboken, NJ, USA: John Wiley and Sons), 1235–1242.
Bonfanti, G., Bitencourt, P. R., Bona, K. S., Silva, P. S., Jantsch, L. B., Pigatto, A. S., et al. (2013). Syzygium Jambos and Solanum Guaraniticum Show Similar Antioxidant Properties but Induce Different Enzymatic Activities in the Brain of Rats. Molecules 18 (8), 9179–9194. doi:10.3390/molecules18089179
Chakravarty, A. K., Das, B., Sarkar, T., Masuda, K., and Shiojima, K. (1998). ChemInform Abstract: Ellagic Acid Derivatives from the Leaves of Eugenia Jambos Linn. ChemInform 30 (25), no. doi:10.1002/chin.199925211
Chua, L. K., Lim, C. L., Ling, A. P. K., Chye, S. M., and Koh, R. Y. (2019). Anticancer Potential of Syzygium Species: a Review. Plant Foods Hum. Nutr. 74 (1), 18–27. doi:10.1007/s11130-018-0704-z
Cock, I. E., and Cheesman, M. (2018). “Bioactive Compounds of Medicinal Plants,” in Bioactive Compounds of Medicinal Plants: Properties and Potential for Human Health. Editors M. R. Goyal, and A. O. Ayeleso (Williston: Apple Academic Press), 35–84.
Daly, J., Hamrick, D., Gary, G., and Guinn, A. (2016). Maize Value Chains in East Africa. London, United Kingdom: International Growth Centre, 1–50.
Deka, K., Kakoti, B. B., and Das, M. (2021). Antiurolithiatic Activity of Leaf Extracts of Syzygium Jambos (l.) Alston and its Zinc Nanoparticles: an In-Vitro and In-Vivo Approach. Int. J. Pharm. Sci. Res. 12 (1), 336–346. doi:10.13040/IJPSR.0975-8232.12(1).336-46
Dhanabalan, R. M. P., and Devakumar, J. (2014). In Vivo antiplasmodial Activity of Four Folklore Medicinal Plants Used Among Tribal Communities of Western Ghats, Coimbatore, Tamil Nadu. J. Pharm. Res. 8 (6), 751–759.
Djipa, C. D., Delmée, M., and Quetin-Leclercq, J. (2000). Antimicrobial Activity of Bark Extracts of Syzygium Jambos (L.) Alston (Myrtaceae). J. Ethnopharmacol. 71 (1-2), 307–313. doi:10.1016/s0378-8741(99)00186-5
Donatini, R. S., Ishikawa, T., Barros, S. B. M., and Bacchi, E. M. (2009). Atividades antiúlcera e antioxidante Do extrato de folhas de Syzygium jambos (L.) Alston (Myrtaceae). Rev. Bras. Farmacogn. 19, 89–94. doi:10.1590/s0102-695x2009000100018
Donatini, R. S., Kato, E., Ohara, M. T., and Bacchi, E. M. (2013). Morphoanatomy and Antimicrobial Study of Syzygium Jambos (L.) Alston (Myrtaceae) Leaves. Lat. Am. J. Pharm. 32 (4), 518.
Dutta, P. P., Bordoloi, M., Gogoi, K., Roy, S., Narzary, B., Bhattacharyya, D. R., et al. (2017). Antimalarial Silver and Gold Nanoparticles: Green Synthesis, Characterization and In Vitro Study. Biomed. Pharmacother. 91, 567–580. doi:10.1016/j.biopha.2017.04.032
Gavillán-Suárez, J., Aguilar-Perez, A., Rivera-Ortiz, N., Rodríguez-Tirado, K., Figueroa-Cuilan, W., Morales-Santiago, L., et al. (2015). Chemical Profile and In Vivo Hypoglycemic Effects of Syzygium Jambos, Costus Speciosus and Tapeinochilos Ananassae Plant Extracts Used as Diabetes Adjuvants in Puerto Rico. BMC Complement. Altern. Med. 15, 244. doi:10.1186/s12906-015-0772-7
Ghareeb, M. A., Hamed, M. M., Abdel-Aleem, A.-a. H., Saad, A. M., Abdel-Aziz, M. S., and Hadad, A. (2017). Extraction, Isolation, and Characterization of Bioactive Compounds and Essential Oil from Syzygium Jambos. Asian J. Pharm. Clin. Res. 10 (8), 194. doi:10.22159/ajpcr.2017.v10i8.18849
Ghareeb, M. A., Saad, A. M., Abdel-Aleem, A. H., Abdel-Aziz, M. S., Hamed, M. M., and Hadad, A. H. (2016). Antioxidant, Antimicrobial, Cytotoxic Activities and Biosynthesis of Silver and Gold Nanoparticles Using Syzygium Jambos Leave Growing in Egypt. Der Pharm. Chem. 8, 277–286.
Guedes, C. M., Pinto, A. B., Moreira, R. F. A., and De Maria, C. A. B. (2004). Study of the Aroma Compounds of Rose Apple (Syzygium Jambos Alston) Fruit from Brazil. Eur. Food Res. Technol. 219 (5), 460–464. doi:10.1007/s00217-004-0967-5
Haque, M. (2015). Investigation of the Medicinal Potentials of Syzygium Jambos (L.) Extract and Characterization of the Isolated Compounds. Am. J. BioScience 3 (2), 12. doi:10.11648/j.ajbio.s.2015030201.13
Harsha, P. V., Ashoka, S. M., Karunakar, H., and Shabaraya, A. R. (2021). Syzygium Jambos: A Brief Review. World J. Pharm. Pharm. Sci. doi:10.20959/wjpps20214-18583
Hossain, H., Rahman, S. E., Akbar, P. N., Khan, T. A., Rahman, M. M., and Jahan, I. A. (2016). HPLC Profiling, Antioxidant and In Vivo Anti-inflammatory Activity of the Ethanol Extract of Syzygium Jambos Available in Bangladesh. BMC Res. Notes 9, 191. doi:10.1186/s13104-016-2000-z
Inostroza-Nieves, Y., Valentin-Berrios, S., Vega, C., Prado, G. N., Luciano-Montalvo, C., Romero, J. R., et al. (2021). Inhibitory Effects of Syzygium Jambos Extract on Biomarkers of Endothelial Cell Activation. Complement. Med. Therapies. doi:10.21203/rs.3.rs-926922/v1
Islam, M. R., Parvin, M. S., and Islam, M. E. (2012). Antioxidant and Hepatoprotective Activity of an Ethanol Extract of Syzygium Jambos (L.) Leaves. Drug Discov. Ther. 6 (4), 205–211. doi:10.5582/ddt.2012.v6.4.205
Jayasinghe, U. L., Ratnayake, R. M., Medawala, M. M., and Fujimoto, Y. (2007). Dihydrochalcones with Radical Scavenging Properties from the Leaves of Syzygium Jambos. Nat. Prod. Res. 21 (6), 551–554. doi:10.1080/14786410601132238
Koagne, R. R., Annang, F., Cautain, B., Martín, J., Pérez-Moreno, G., Bitchagno, G. T. M., et al. (2020). Cytotoxycity and Antiplasmodial Activity of Phenolic Derivatives from Albizia Zygia (DC.) J.F. Macbr. (Mimosaceae). BMC Complement. Med. Ther. 20 (1), 8. doi:10.1186/s12906-019-2792-1
Koteshwara, A., Sakander, H., and Akhilesh, B. (2015). Evaluation of Antifungal Potential of Selected Medicinal Plants against Human Pathogenic Fungi. Int. J. Green. Pharm. 9 (2), 110–117. doi:10.4103/0973-8258.155058
Kuiate, J. R., Mouokeu, S., Wabo, H. K., and Tane, P. (2007). Antidermatophytic Triterpenoids from Syzygium Jambos (L.) Alston (Myrtaceae). Phytother. Res. 21 (2), 149–152. doi:10.1002/ptr.2039
Li, G. Q., Zhang, Y. B., Wu, P., Chen, N. H., Wu, Z. N., Yang, L., et al. (2015). New Phloroglucinol Derivatives from the Fruit Tree Syzygium Jambos and Their Cytotoxic and Antioxidant Activities. J. Agric. Food Chem. 63 (47), 10257–10262. doi:10.1021/acs.jafc.5b04293
Lin, D. D., Liu, J. W., Li, W. G., Luo, W., Cheng, J. L., and Chen, W. W. (2014). Chemical Constituents from Stems of Syzygium Jambos Var. Jambos and Their In Vitro Cytotoxicity. Chin. Trad. Herb. Drugs 45 (17), 1993–1997. doi:10.7501/j.issn.0253-2670.2014.14.006
Luciano-Montalvo, C., Boulogne, I., and Gavillán-Suárez, J. (2013). A Screening for Antimicrobial Activities of Caribbean Herbal Remedies. BMC Complement. Altern. Med. 13, 126. doi:10.1186/1472-6882-13-126
Mabberley, D. J. (2017). Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses. 4 éd. Cambridge, United Kingdom: Cambridge University Press.
Mahmoud, M. F., Abdelaal, S., Mohammed, H. O., El-Shazly, A. M., Daoud, R., El Raey, M. A., et al. (2021). Syzygium Jambos Extract Mitigates Pancreatic Oxidative Stress, Inflammation and Apoptosis and Modulates Hepatic IRS-2/AKT/GLUT4 Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 142, 112085. doi:10.1016/j.biopha.2021.112085
Mangini, L. F. K., Valt, R. B. G., Ponte, M. J. J. d. S., and Ponte, H. d. A. (2020). Vanadium Removal from Spent Catalyst Used in the Manufacture of Sulfuric Acid by Electrical Potential Application. Separat. Purif. Technol. 246, 116854. doi:10.1016/j.seppur.2020.116854
Maskey, K., and Shah, B. B. (1982). Sugars in Some Nepalese Edible Wild Fruits. J. Nepal Chem. Soc. 2, 23–30.
M. Khalaf, O., Abdel-Aziz, M. S., El-Hagrassi, A. M., Osman, A. F., and Ghareeb, M. A. (2021). Biochemical Aspect, Antimicrobial and Antioxidant Activities of Melaleuca and Syzygium Species (Myrtaceae) Grown in Egypt. J. Phys. Conf. Ser. 1879 (2), 022062. doi:10.1088/1742-6596/1879/2/022062
Mohanty, S., and Cock, I. E. (2010). Bioactivity of Syzygium Jambos Methanolic Extracts: Antibacterial Activity and Toxicity. Pharmacognosy Res. 2 (1), 4–9. doi:10.4103/0974-8490.60577
Murugan, S., Devi, P. U., Parameswari, N. K., and Mani, K. R. (2011). Antimicrobial Activity of Syzygium Jambos against Selected Human Pathogens. Int. J. Pharm. Pharm. Sci. 3 (2), 44–47.
Musthafa, K. S., Sianglum, W., Saising, J., Lethongkam, S., and Voravuthikunchai, S. P. (2017). Evaluation of Phytochemicals from Medicinal Plants of Myrtaceae Family on Virulence Factor Production by Pseudomonas aeruginosa. Apmis 125 (5), 482–490. doi:10.1111/apm.12672
Nawwar, M. A., Hashem, A. N., Hussein, S. A., Swilam, N. F., Becker, A., Haertel, B., et al. (2016). Phenolic Profiling of an Extract from Eugenia Jambos L. (Alston)-Tthe Structure of Three Flavonoid Glycosides-Aantioxidant and Cytotoxic Activities. Pharmazie 71, 162–168. doi:10.1691/ph.2016.5747
Nesa, F., Shoeb, M., Islam, M. M., and Islam, M. N. (2021). Studies of Physico-Chemical Properties and Cytotoxicity of Fruits of Syzygium Jambos L. Against HeLa and Vero Cell Lines. Bangla Pharma J. 24 (2), 111–116. doi:10.3329/bpj.v24i2.54709
Noé, W., Murhekar, S., White, A., Davis, C., and Cock, I. E. (2019). Inhibition of the Growth of Human Dermatophytic Pathogens by Selected Australian and Asian Plants Traditionally Used to Treat Fungal Infections. J. Mycol. Med. 29 (4), 331–344. doi:10.1016/j.mycmed.2019.05.003
Panthong, K., and Voravuthikunchai, S. P. (2020). Eugejambones A−D from Leaves of Eugenia Jambos. Phytochemistry Lett. 38, 49–54. doi:10.1016/j.phytol.2020.05.011
Rajkumari, J., Dyavaiah, M., Sudharshan, S. J., and Busi, S. (2018b). Evaluation of In Vivo Antioxidant Potential of Syzygium Jambos (L.) Alston and Terminalia Citrina Roxb. Towards Oxidative Stress Response in Saccharomyces cerevisiae. J. Food Sci. Technol. 55 (11), 4432–4439. doi:10.1007/s13197-018-3355-z
Rajkumari, J., Borkotoky, S., Murali, A., and Busi, S. (2018a). Anti-Quorum Sensing Activity of Syzygium Jambos (L.) Alston against Pseudomonas aeruginosa PAO1 and Identification of its Bioactive Components. South Afr. J. Bot. 118, 151–157. doi:10.1016/j.sajb.2018.07.004
Reddy, Y. N., Vinil Kumar, V., and Naresh Chandra, R. N. B. S. (2014). In Vitro antioxidant and Anti-inflammatory Activity of Hydro Methanolic Extract of Leaves of Syzygium Jambos (L) Alston. Int. J. Pharm. Life Sci. 2 (2), 71–82.
Reis, A. S., Silva, L. de. S., Martins, C. F., and de Paula, J. R. (2021). Analysis of the Volatile Oils from Three Species of the Gender Syzygium. Res. Soc. Dev. 10 (7), e13510716375. doi:10.33448/rsd-v10i7.16375
Reynertson, K. A., Yang, H., Jiang, B., Basile, M. J., and Kennelly, E. J. (2008). Quantitative Analysis of Antiradical Phenolic Constituents from Fourteen Edible Myrtaceae Fruits. Food Chem. 109 (4), 883–890. doi:10.1016/j.foodchem.2008.01.021
Rocchetti, G., Lucini, L., Ahmed, S. R., and Saber, F. R. (2019). In Vitro cytotoxic Activity of Six Syzygium Leaf Extracts as Related to Their Phenolic Profiles: An Untargeted UHPLC-QTOF-MS Approach. Food Res. Int. 126, 108715. doi:10.1016/j.foodres.2019.108715
Selvam, N. T., Venkatakrishnan, V., Dhamodharan, R., Murugesan, S., and Kumar, S. D. (2013). Hepatoprotective Activity of Methanolic Extract of Syzygium Jambos (Linn.) Leaf against Paracetamol Intoxicated Wistar Albino Rats. Ayu 34 (3), 305–308. doi:10.4103/0974-8520.123133
Sharma, R., Kishore, N., Hussein, A., and Lall, N. (2013). Antibacterial and Anti-inflammatory Effects of Syzygium Jambos L. (Alston) and Isolated Compounds on Acne Vulgaris. BMC Complement. Altern. Med. 13 (1), 292. doi:10.1186/1472-6882-13-292
Slowing, K., Söllhuber, M., Carretero, E., and Villar, A. (1994). Flavonoid Glycosides from Eugenia Jambos. Phytochemistry 37 (1), 255–258. doi:10.1016/0031-9422(94)85036-4
Slowing, K., Carretero, E., and Villar, A. (1996). Anti-inflammatory Compounds of Eugenia Jambos. Phytother. Res. 10 (1), 126–127.
Sobeh, M., Braun, M. S., Krstin, S., Youssef, F. S., Ashour, M. L., and Wink, M. (2016). Chemical Profiling of the Essential Oils of Syzygium Aqueum, Syzygium Samarangense and Eugenia Uniflora and Their Discrimination Using Chemometric Analysis. Chem. Biodivers. 13 (11), 1537–1550. doi:10.1002/cbdv.201600089
Sobeh, M., Esmat, A., Petruk, G., Abdelfattah, M. A. O., Dmirieh, M., Monti, D. M., et al. (2018). Phenolic Compounds from Syzygium Jambos (Myrtaceae) Exhibit Distinct Antioxidant and Hepatoprotective Activities In Vivo. J. Funct. Foods 41, 223–231. doi:10.1016/j.jff.2017.12.055
Subbulakshmi, K., Satish, S., and Shabaraya, A. R. (2021). Rose Apple Fruit: A Pharmacological Review. World J. Pharm. Pharm. Sci. 10, 842–849. doi:10.20959/wjpps20214-18707
Sun, Z., Huang, Q., and Feng, C. (2020). Complete Chloroplast Genome Sequence of the Rose Apple, Syzygium Jambos (Myrtaceae). Mitochondrial DNA B 5 (3), 3460–3462. doi:10.1080/23802359.2020.1826000
Tamiello, C. S., Adami, E. R., de Oliveira, N. M. T., Acco, A., Iacomini, M., and Cordeiro, L. M. C. (2018a). Structural Features of Polysaccharides from Edible Jambo (Syzygium Jambos) Fruits and Antitumor Activity of Extracted Pectins. Int. J. Biol. Macromol. 118, 1414–1421. doi:10.1016/j.ijbiomac.2018.06.164
Tamiello, C. S., do Nascimento, G. E., Iacomini, M., and Cordeiro, L. M. C. (2018b). Arabinogalactan from Edible Jambo Fruit Induces Different Responses on Cytokine Secretion by THP-1 Macrophages in the Absence and Presence of Proinflammatory Stimulus. Int. J. Biol. Macromol. 107, 35–41. doi:10.1016/j.ijbiomac.2017.08.148
Teixeira, C. C., Fuchs, F. D., Blotta, R. M., Knijnik, J., Delgado, I. C., Netto, M. S., et al. (1990). Effect of tea Prepared from Leaves of Syzygium Jambos on Glucose Tolerance in Nondiabetic Subjects. Diabetes Care 13 (8), 907–908. doi:10.2337/diacare.13.8.907
Thamizh Selvam, N., Acharya, M., Venkatakrishnan, V., and Murugesan, S. (2016). Effect of Methanolic Extract of (Linn.) Alston Leaves at Intra Syzygium Jambos Cellular Level in Selective Liver Cancer Cell Line: Molecular Approach for its Cytotoxic Activity. Adv. Pharm. J. 1 (5), 139.
Ticona, L. A., Pérez, B. S., Alejano, V. M., and Slowing, K. (2021). Anti-inflammatory and Anti-arthritic Activities of Glycosylated Flavonoids from Syzygium Jambos in Edematogenic Agent-Induced Paw Edema in Mice. Rev. Bras. Farmacogn. 31, 429–441. doi:10.1007/s43450-021-00167-0
Twilley, D., Langhansová, L., Palaniswamy, D., and Lall, N. (2017). Evaluation of Traditionally Used Medicinal Plants for Anticancer, Antioxidant, Anti-inflammatory and Anti-viral (HPV-1) Activity. South Afr. J. Bot. 112, 494–500. doi:10.1016/j.sajb.2017.05.021
Vagula, J. M., Visentainer, J. V., Lopes, A. P., Maistrovicz, F. C., Rotta, E. M., and Suzuki, R. M. (2019). Antioxidant Activity of Fifteen Seeds from Fruit Processing Residues by Different Methods. Acta Sci. Technol. 41, e35043. doi:10.4025/actascitechnol.v41i2.35043
van Wyk, B-E., and Wink, M. (2015). Phytomedicines, Herbal Drugs, and Poisons. Chicago: The University of Chicago Press.
Vernin, G., Vernin, G., Metzger, J., Roque, C., and Pieribattesti, J.-C. (1991). Volatile Constituents of the Jamrosa AromaSyzygium jambosL. Aston from Reunion Island. J. Essent. Oil Res. 3 (2), 83–97. doi:10.1080/10412905.1991.9697916
Wamba, B. E. N., Nayim, P., Mbaveng, A. T., Voukeng, I. K., Dzotam, J. K., Ngalani, O. J. T., et al. (2018). Syzygium Jambos Displayed Antibacterial and Antibiotic-Modulating Activities against Resistant Phenotypes. Evid. Based Complement. Alternat. Med. 2018, 5124735. doi:10.1155/2018/5124735
Wen, Z., Ling, M., Yu, S., Zhuang, Y., Luo, X., Pan, Z., et al. (2019). Study on Inhibitory Effects of Ethanol Extract of Different Medicinal Parts from Syzygium Jambos on the Activities of α-Glycosidase and α-Amylase. China Pharm., 3246–3251.
Wong, K. C., and Lai, F. Y. (1996). Volatile Constituents from the Fruits of FourSyzygium Species Grown in Malaysia. Flavour Fragr. J. 11 (1), 61–66. doi:10.1002/(sici)1099-1026(199601)11:1<61:aid-ffj539>3.0.co;2-1
Yang, L. L., Lee, C. Y., and Yen, K. Y. (2000). Induction of Apoptosis by Hydrolyzable Tannins from Eugenia Jambos L. On Human Leukemia Cells. Cancer Lett. 157 (1), 65–75. doi:10.1016/S0304-3835(00)00477-8
Yunus, S. N. M., Abas, F., Jaafar, A. H., Azizan, A., Zolkeflee, N. K. Z., and Abd Ghafar, S. Z. (2021). Antioxidant and α-glucosidase Inhibitory Activities of Eight Neglected Fruit Extracts and UHPLC-MS/MS Profile of the Active Extracts. Food Sci. Biotechnol. 30 (2), 195–208. doi:10.1007/s10068-020-00856-x
Keywords: Syzygium jambos, medicinal plants, pharmacological activities, antioxidant, antiinflammatory
Citation: Ochieng MA, Ben Bakrim W, Bitchagno GTM, Mahmoud MF and Sobeh M (2022) Syzygium jambos L. Alston: An Insight Into its Phytochemistry, Traditional Uses, and Pharmacological Properties. Front. Pharmacol. 13:786712. doi: 10.3389/fphar.2022.786712
Received: 30 September 2021; Accepted: 03 January 2022;
Published: 24 January 2022.
Edited by:
Jules-Roger Kuiate, University of Dschang, CameroonReviewed by:
Subhalakshmi Ghosh, Independent Researcher, Kolkata, IndiaNjayou Frederic Nico, University of Yaounde I, Cameroon
Copyright © 2022 Ochieng, Ben Bakrim, Bitchagno, Mahmoud and Sobeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabin Thierry M. Bitchagno, gabin.bitchagno@um6p.ma; Mansour Sobeh, mansour.sobeh@um6p.ma
†These authors have contributed equally to this work
 Melvin Adhiambo Ochieng
Melvin Adhiambo Ochieng Widad Ben Bakrim
Widad Ben Bakrim Gabin Thierry M. Bitchagno
Gabin Thierry M. Bitchagno Mona F. Mahmoud
Mona F. Mahmoud Mansour Sobeh
Mansour Sobeh