Study elucidates molecular mechanism that controls a critical step in the meiotic division of egg cells
When producing our germ cells—egg and sperm—a special feature is required in the cell division process: Since male and female germ cells fuse during fertilization, the chromosome set of our genetic material, which is normally present in duplicate, must first be halved. Otherwise, the chromosomes would double with each fertilization, resulting in serious consequences for the embryo. This particular form of cell division in germ cells is called maturation division or meiosis and takes place in two steps, meiosis I and meiosis II, in which the chromosome set is halved according to a specific pattern.
"In the development of egg cells, the process is even a little more complex than in sperm, because the maturation division must be halted twice: once in meiosis I and once in meiosis II," explains Thomas Mayer, professor of molecular genetics at the University of Konstanz. Both arrests are regulated in different ways and differ in their function.
The first allows the nascent egg cell to grow and absorb nutrients; during the second arrest, the now matured egg awaits fertilization. "The second arrest thus prevents the embryo from developing from an unfertilized egg. It is therefore of enormous importance for our reproduction and its proper timing is essential," Mayer adds. How exactly this second arrest is controlled, in particular how it is prevented from occurring prematurely in the first meiotic division, was not known until now.
Key role of a hitherto little-studied protein
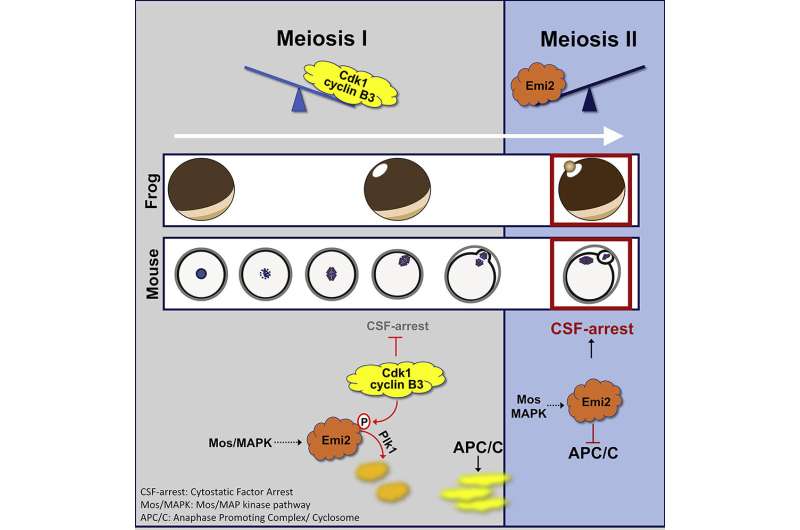
Together with colleagues from the Sorbonne Université in Paris, Mayer and his team have now succeeded in shedding some light on the subject. With their recent publication in Developmental Cell, they are the first to describe the interaction of the two proteins Cyclin B3 and Emi2 and their effect on the timeline of the process. Cyclin B3 is found exclusively in egg cells and has been understudied to date. Emi2, on the other hand, was already known to be directly responsible for the arrest in meiosis II: If it is present in a sufficient quantity, the maturation division is halted.
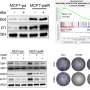
Using frog and mouse egg cells, the researchers have now been able to show that Cyclin B3 keeps the availability of Emi2 below this critical threshold during the first maturation division by causing—in conjunction with another protein—its degradation. This prevents the arrest from occurring prematurely. At the end of meiosis I, Cyclin B3 is then degraded itself. Consequently, without the presence of cyclin B3 during meiosis II, the availability of Emi2 increases until there is enough to halt the second maturation division at just the right moment.
A complete loss of Cyclin B3 in both frog and mouse egg cells leads to a premature, Emi2-mediated arrest in meiosis I, as the researchers demonstrated in their experiments. "The similarity of the processes between frogs and mice suggests that this function of cyclin B3, which is of great importance for our reproduction, emerged early in vertebrate evolution and has remained unchanged since then," concludes Mayer. This assumption is supported by the fact that women who have a mutation in Cyclin B3 suffer from recurrent miscarriages.
More information: Nora Bouftas et al, Cyclin B3 implements timely vertebrate oocyte arrest for fertilization, Developmental Cell (2022). DOI: 10.1016/j.devcel.2022.09.005
Journal information: Developmental Cell
Provided by University of Konstanz