Crystallography
Syllabus
................................................................................................................................................
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics.
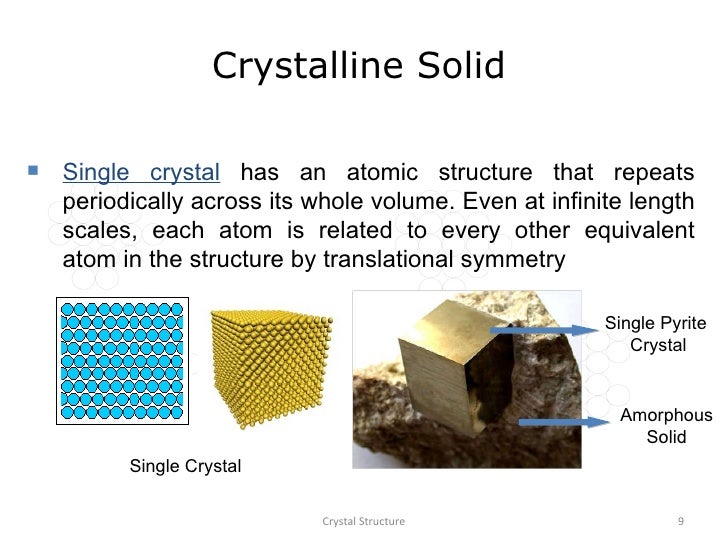
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions.
In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography.
The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification.
Microscopically, a single crystal has atoms in a near-perfect periodic arrangement; a polycrystal is composed of many microscopic crystals (called "crystallites" or "grains"); and an amorphous solid (such as glass) has no periodic arrangement even microscopically.
In materials science, a single crystal (or single-crystal solid or monocrystalline solid) is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries.[1] The absence of the defects associated with grain boundaries can give monocrystals unique properties, particularly mechanical, optical and electrical, which can also be anisotropic, depending on the type of crystallographic structure.
The orientation of crystallites can be random with no preferred direction, called random texture, or directed, possibly due to growth and processing conditions. While the structure of a (single) crystal is highly ordered and its lattice is continuous and unbroken, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in-between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are polycrystalline, made of a large number crystallites held together by thin layers of amorphous solid. Most inorganic solids are polycrystalline, including all common metals, many ceramics, rocks, and ice.
An amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymously with amorphous solid; however, these terms refer specifically to amorphous materials that undergo a glass transition.[1] Examples of amorphous solids include glasses, metallic glasses, and certain types of plastics and polymers.


What is Crystal Structure?
A crystal structure is made of atoms. A crystal lattice is made of points. A crystal system is a set of axes. In other words, the structure is an ordered array of atoms, ions or molecules.
Crystal Structure is obtained by attaching atoms, groups of atoms or molecules. This structure occurs from the intrinsic nature of the constituent particles to produce symmetric patterns. A small group of a repeating pattern of the atomic structure is known as the unit cell of the structure. A unit cell is the building block of the crystal structure and it also explains in detail the entire crystal structure and symmetry with the atom positions along with its principal axes. The length, edges of principal axes and the angle between the unit cells are called lattice constants or lattice parameters.
Seven crystal system:
Crystal system is a method of classifying crystalline substances on the basis of their unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, trigonal (also known as rhombohedral), orthorhombic, monoclinic and triclinic.

...............
· Copper K-α is an x-ray energy frequently used on labscale x-ray instruments. The energy is 8.04 keV, which corresponds to an x-ray wavelength of 1.5406 Å.
Packing Efficiency
Packing efficiency is defined as the percentage of space occupied by constituent particles packed inside the lattice. It can be calculated with the help of geometry in three structures namely:
Body-Centred Cubic Structures
In body-centred cubic structures, the three atoms are diagonally arranged. To find the packing efficiency we consider a cube with edge length a, face diagonal length b and cube diagonal as c.
It is a great experience having the slides even after lecture period.Thank you sir!
ReplyDeletefeels like we are way advanced sir. thank you sir for this opportunity.
ReplyDeleteThe most interesting text on this interesting topic that can be found on the net ... Clonazepam
ReplyDeleteI should say only that its awesome! The blog is informational and always produce amazing things. mental health
ReplyDeletethanks for sharing nice post health is a big wealth
ReplyDeleteThank you for taking the time to publish this information very useful! small flower vase
ReplyDeletebuy cocaine online High Purity Research Chemicals For Any Industry shop now! We provide high quality Bath Salt And Herbal Incense with fast delivery. We sell in bulks too.
ReplyDelete