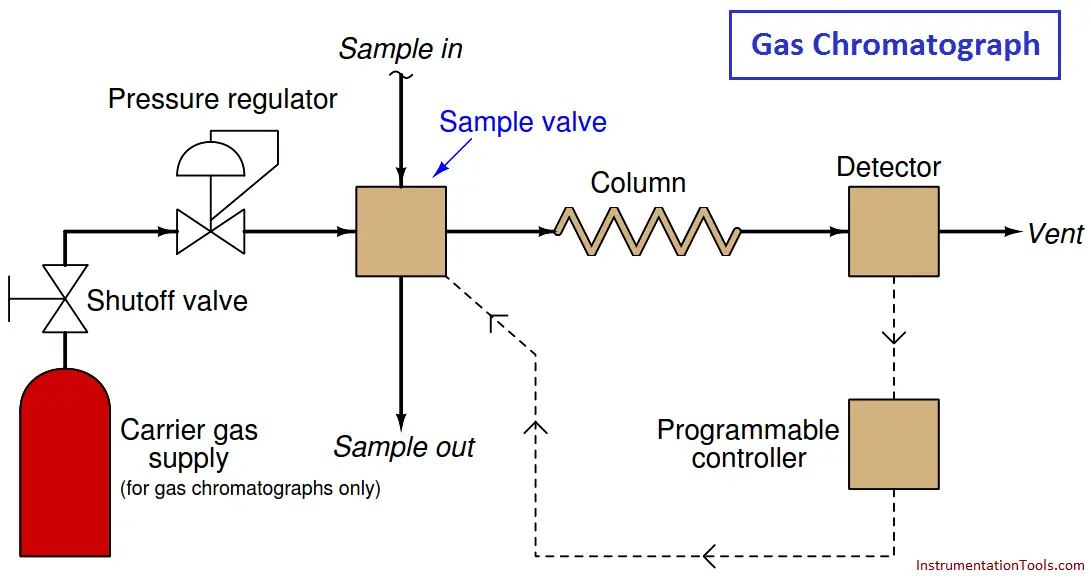
The most common type of chromatography used in continuous process analysis is the gas chromatograph (abbreviated “GC”), so named because the mobile phase is a gas (or a vapor ( Note 1 ) rather than a liquid.
In a GC, the sample to be analyzed is injected at the head of a very long and very narrow tube packed with solid and/or liquid material (Note 2 ). This long and narrow tube (called the column) is designed to impede the passage of the sample molecules.
A continuous flow of “carrier gas” washes the sample compounds down the length of the column, allowing them to separate over time according to how they interact with the stationary phase packed inside the column.
Note 1 : Gas chromatographs are commonly used for industrial analysis on liquid sample streams, by using a heater at the inlet of the chromatograph to vaporize the liquid sample prior to analysis. In such applications the column and sample valve(s) must be maintained in a heated condition as well so that the sample does not condense back into liquid form during the analysis.
Note 2 : Stationary phase material used in many hydrocarbon GC’s looks much like oily sand.
Gas Chromatograph
A simplified schematic of a process GC shows how it functions:
The sample valve periodically injects a very precise quantity of sample into the entrance of the column tube and then shuts off to allow the constant-flow carrier gas to wash this sample through the length of the column tube.
Each chemical species within the sample travels through the column at different rates, exiting the column at different times. All the detector needs to do is be able to tell the difference between pure carrier gas and carrier gas mixed with anything else.
An important detail to note here is that the detector need not be discriminatory in its response to different chemical compounds, with just one exception: it need only distinguish between carrier and non-carrier compounds.
The ability for an analyzer to measure singular compounds out of a mixture is the fundamental challenge of all analytical instrumentation, and here we see the genius of chromatography: the variable we use to discriminate between different chemical compounds within the sample is retention time through the column, and nothing else.
Rather than rely on some clever sensor technology to selectively detect one chemical compound out of a mixture, we are able to use a non-specific sensor and let time be the discriminating variable.
Appealing to the marathon analogy again, it’s as if a platform weigh-scale is situated at the finish line, to weigh the runners as they finish the race. The scale cannot discern the running speed of any runner by their measured weight – indeed, all the scale indicates is that someone is crossing the finish line – but it can determine the running speed based on when the runner steps on the scale.
If we plot the response of a chromatograph’s detector on a graph, we see a pattern of peaks, each one indicating the departure of a species “group” (i.e. a different chemical compound) exiting the column. This graph is typically called a chromatogram:
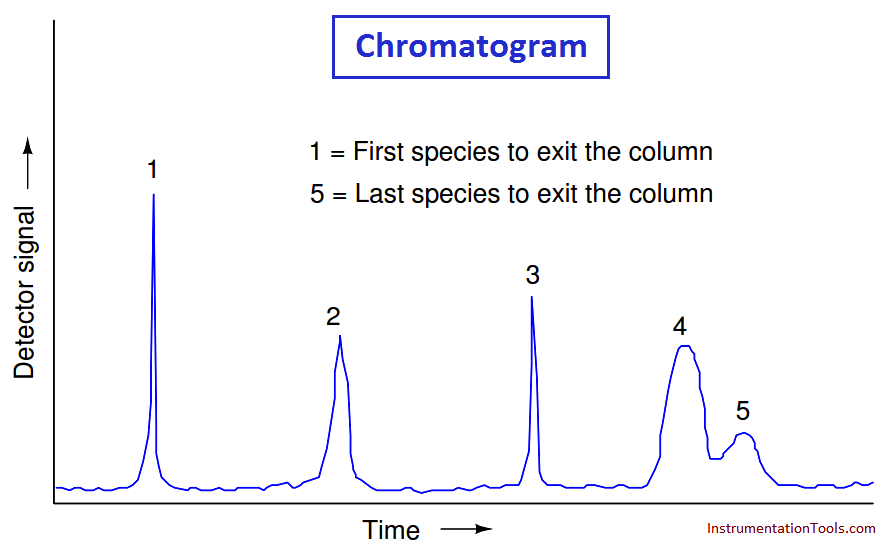
Narrow peaks represent compact bunches of molecules all exiting the column at nearly the same time, analogous to a group of runners crossing the finish line running shoulder-to-shoulder, all stepping on and off the platform scale at the same time.
Wide peaks represent more diffuse groupings of similar (or identical) molecules, analogous to a group of equally-fast runners crossing the finish line as a diffuse group. In this chromatogram, you can see that species 4 and 5 are not clearly differentiated over time.
Better separation of species may be achieved by altering the sample volume, carrier gas flow rate, carrier gas pressure, type of carrier gas, column packing material, and/or column temperature.
Different Gases identification
Recall that the purpose of any analytical instrument is to discriminately measure one variable or component concentration amongst a mix of different chemical components.
The fundamental challenge of chemical analysis is how to reliably and accurately detect that one “species” of chemical while being unaffected by the presence of any other chemical species.
Most analyzers rely on a sensor that exploits some unique characteristic of the chemical of interest (e.g. its unique ability to pass through a special membrane, its ability to absorb a unique spectrum of light, its tendency to engage in a unique chemical reaction, etc.), but not chromatographs.
Chromatographs simply delay the flow rate of different chemical species through a long tube based on those species’ mass and interactions with the stationary phase, using time as the discriminating variable.
This allows chromatographs to be nearly universal in the range of chemical species they can measure, while using relatively simple and uncomplicated detectors. The detector need only discriminate between carrier gas and anything that isn’t carrier gas in order to serve its purpose in a gas chromatograph.
Identifying a suitable detector technology is relatively easy given that fact that the type of carrier gas used in a GC is something chosen by the designer of the GC.
In other words, chromatography gives one the freedom to choose a compound that’s easy for a sensor to detect, rather than try to find a sensor able to detect the specific species/gases one needs to measure in the process. This is important given the fact that there are many more types of chemical compounds in the world than there are chemical sensor types!
In general, chemical compounds having less molecular weight tend to exit the column earlier, while compounds having greater molecular weight exit the column later.
The precise sequence of species elution through a column depends greatly on the stationary phase material type, as well as the carrier fluid type. Proper selection of stationary phase and carrier compounds is essential to efficient chromatograph operation, and is usually the domain of trained chemists.
CLICK HERE to see animation showing how a chromatograph separates a sample into its constituent species/gases.
Since species (gases) identification in a chromatograph is performed with time as the discriminating factor, a chromatograph’s ability to accurately identify chemical compounds depends on its control computer “knowing” when to expect various compounds to exit the column.
Chromatographs are calibrated by injecting a sample containing known concentrations of the species of interest (as well as any potentially interfering species), then timing the beginnings and ends of each species peak as each substance exits the column.