Understanding lattices: https://pubs.acs.org/doi/10.1021/acs.jchemed.9b01207
Crystals are solid-state bodies, that are characterized by three criteria:
- homogeneous in all direction
- anisotropic: properties (e.g. hardness, conductivity) are different for different directions
- 3D periodically ordered
The opposite of a crystal are amorphous materials.
Correspondence principle: the phenotype/shape of a crystal is related to its molecular make-up
Unit cell: minimal unit, that contains all symmetry elements of the crystal and builds up the whole crystal structure only by repeated translations along all three spatial directions (no rotation allows)
Chemical composition ratio of the unit cell is the same as for the whole crystal.
All unit cells are Parallelepiped: six parallelograms, of which two of each are congruent (superimposable) and lie in parallal planes
Metric of the unit cell: 6 cell/lattice parameters:
- length of the edges (cell constants) : a, b, c
- angles between edges (cell angles): α, β, γ
The all crystals can elementarily be described in 7 crystal systems. These and their accompanied symmetries are intrinsic properties of the crystal lattice.
| Name | a | b | c | α | β | γ | Cell constants | Cell angles |
| triclinic | a | b | c | α | β | γ | none | none |
| monoclinic | a | b | c | 90° | β | 90° | none | α = γ = 90° |
| orthorhombic | a | b | c | 90° | 90° | 90° | none | α = β = γ = 90° |
| tetragonal | a | a | c | 90° | 90° | 90° | a = b | α = β = γ = 90° |
| trigonal | a | a | c | 90° | 90° | 120° | a = b | α = β = 90°; γ = 120° |
| hexagonal | a | a | c | 90° | 90° | 120° | a = b | α = β = 90°; γ = 120° |
| cubic | a | a | a | 90° | 90° | 90° | a = b = c | α = β = γ = 90° |
These cell parameters only give indications of the underlying symmetry, they don’t determine the symmetry. For a = b = 4, c = 4.1 and α = β = γ = 90°, it could be tetragonal, orthorhombic, monoclinic or triclinic. So, we cannot determine a crystal system simply based on the the symmetry parameters.
Crystal structure = lattice + motif/base
Lattice: each unit (cell) with same surroundings, which is characterized by its translation vectors (and translation only), which span the unit cells in all directions to give rise to the whole structure:
Motif/base: arrangement of building blocks (atoms) of a unit cell; represented by lattice point
Describing the morphology of the crystal:
- “Tracht” (costume): total set of faces (number and composition of faces of the outer limiting plances of a crystal
- Habitus: relative face development (relative sizes of each face)
- isometric: same dimensions in all directions
https://www.mima.museum/cinderella1.php
Miller indices
Explanatory website: https://www.doitpoms.ac.uk/tlplib/miller_indices/prereqs.php
Lattice planes: family of parallel planes, which intersect the Bravais lattice and are periodic; these planes are fictitious (i.e. don’t represent physical contacts or something) and are only there to connect lattice points and bring a bit order
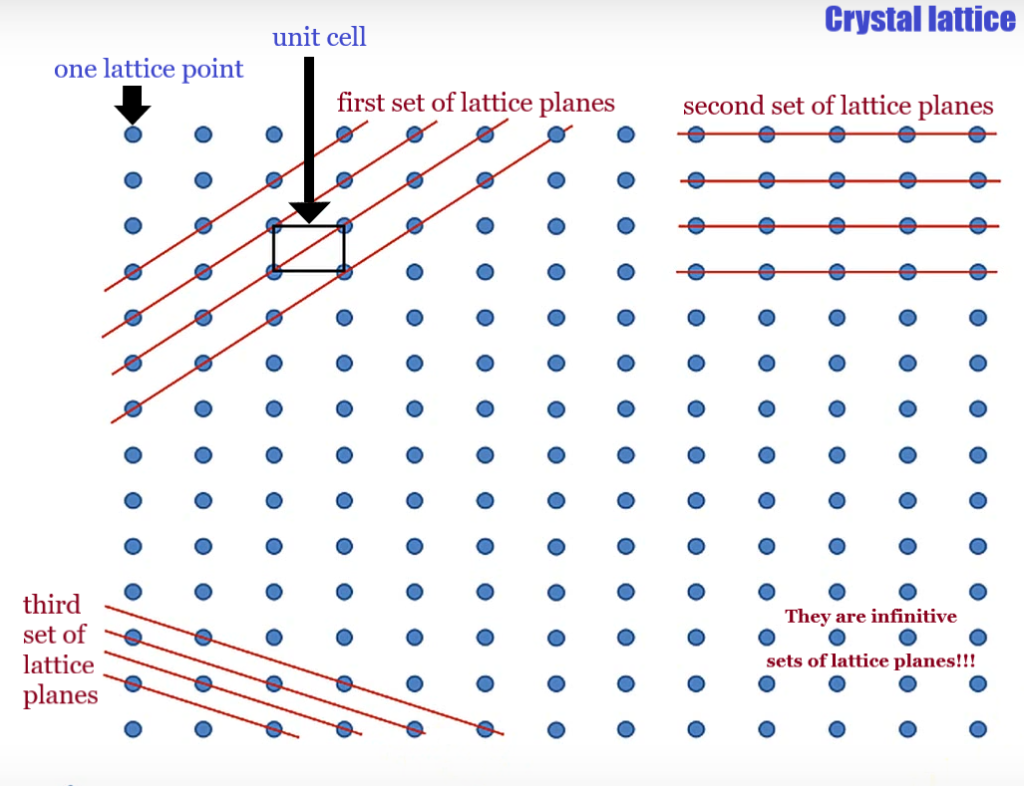
1. set of lattice planes: (1 -1 0)
2. set of lattice planes: (0 1 0)
3. set of lattice planes: (1 2 0)
Miller indices (h k l) is a notation system are used to describe such planes.
Miller indices are given by answering the following question: In how many fractions do the lattice planes intersect the respective lattice constants?
- Identify the unit cell and identify the origin of the cell.
- Go along the vector of the first constant (direction of vector a).
- Count how many lines go through this direction? This is the h value.
- Do the same for the second (b -> k) and third constant (c -> l).
- negative Miller indices are drawn with a bar above the letter: to find out if a Miller index is negative:
- Look at the the Lattice plane that runs through the origin of the unit cell (in red)
- Now go to the next lattice plane along the positive a axis and follow that lattice plane back towards the origin of the cell.
- If the the lattice plane thereby intersects with the positive b axis, the value of k is positive
- if the the lattice plane thereby intersects with the negative b axis, the value of k is negative

Generalization and important aspects:

Why are Miller indices important?
The composition and order of the unit cells at the faces of a crystal can be described by the Miller indices, that describe the lattice planes.

14 Bravais Lattices
The 7 crystal systems described above are given rise to primitive lattices. These are characterized by:
- lattice points only at every corner of the unit cell, but not inside the cell or at the faces
- the unit cell of primitive lattices comprise exactly 1 motif/base, i.e. if each of the 8 corners of the unit cell is thought as a ball, which is cut by the lattice planes into parts of that ball, then the sum all these ball parts in these primitive lattices make up one whole ball
- the primitive unit cell is the smallest possible unit cell
Choosing the unit cell is a completely arbitrary choice, everything that conforms to the definition above can be stated as a unit cell. However, in order to make the work with crystals a bit easy, here are some rules on how to choose a unit cell:
- unit cell should be as small as possible and this means the lattice vectors should be as short as possible
- unit cell should also represent the symmetry of the lattice! And this means the lattice vectors should run parallel to symmetry axes or perpendicular to symmetry planes
- axes should be orthogonal (or hexagonal), so that we the highest possible symmetry
Sometimes, primitive unit cells as the smallest possible unit cell don’t conform to that symmetry indication. Therefore, it can be advantageous not to choose the smallest possible unit cell, in order to describe the crystal in a higher symmetric system of coordinates.
Centered unit cell: Primitive unit cell + additional central lattice point
Forms of centering in unit cells (UC)
| Name | Symbol | Explanation | Nummer of lattice points |
| PrimitiveUC | P | no centering | 1 |
| Single-side face-centeredUC | C(AB) | additional lattice point at one of the faces (in the C-plane or AB-spaned plane) | 2 |
| body-centeredUC | I | additional lattice point exactly inside the unit cell | 2 |
| all-side face-centered unit cell | F | additional lattice point at all faces | 4 |
The 14 Bravais lattice types include all kind of sensible centerings in all 7 crystal classes (redandency and non-compatible symmetry removed).

The symmetry that characterizes each crystal class is still respected in all 14 existing Bravais lattices.
Each atom inside in a unit cell can be described in relation to the unit cell parameters.
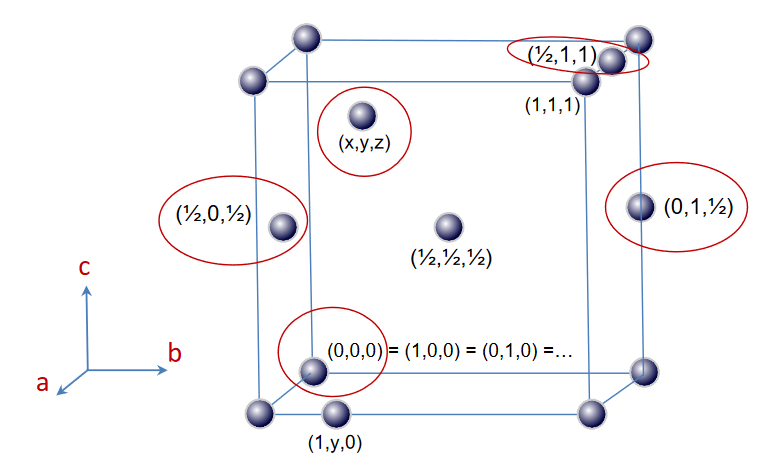
This is taken from the wonderful MOOC on Crystallography from Frank Hoffmann from the University of Hamburg that is accompanied by his blog:
- Youtube-Channel: https://www.youtube.com/channel/UCts9FTFNInqTMvcFpdyap7w/playlists
- WordPress-Blog: https://crystalsymmetry.wordpress.com/

